Can TMS Be a Bridge When Medication Isn’t Working?

What is “treatment-resistant depression” (TRD)?
Clinically, TRD is commonly defined as depression that has not sufficiently responded to an adequate trial of two different antidepressants (from at least two different classes) given at therapeutic doses and durations. Some insurers and clinical guidelines use this “two-trial” rule when deciding whether to cover advanced treatments like TMS. Medicare and commercial payers may have slightly different wording (for example, Medicare sometimes requires one failed antidepressant; Anthem often requires two). The key idea is consistent: TRD means standard medication strategies haven’t produced an acceptable response.
Why do medications sometimes fail?
Antidepressants help many people, but there are several reasons they may not work for someone:
- Different biology: Antidepressants act primarily on neurotransmitter systems (serotonin, norepinephrine, dopamine). Depression is heterogeneous - for some people, symptoms reflect maladaptive brain-circuit function that medications don’t adequately correct.
- Side effects & tolerability: Some people cannot tolerate the side effects of medications at effective doses.
- Partial or transient responses: A medication may help a little (or briefly) but not produce sustained remission.
- Complex or comorbid conditions: Co-occurring anxiety, PTSD, medical conditions or bipolar spectrum features can blunt medication response.
Because these issues are common, clinicians consider alternative mechanisms of treatment - therapies that act on the brain in different ways. TMS is one of those options. (Ketamine/esketamine is another; the treatments can be complementary rather than mutually exclusive.)
How is TMS different from medication?
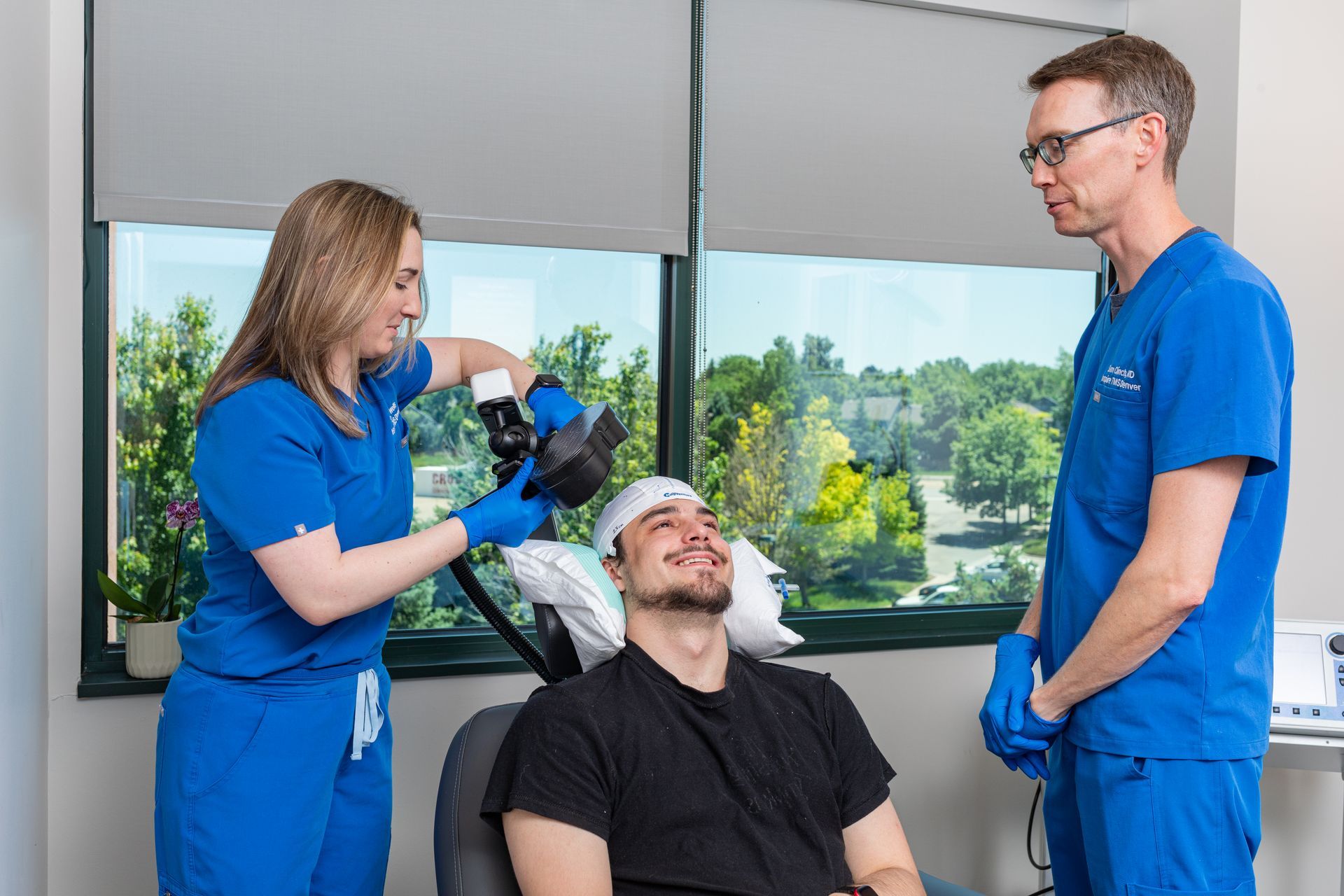
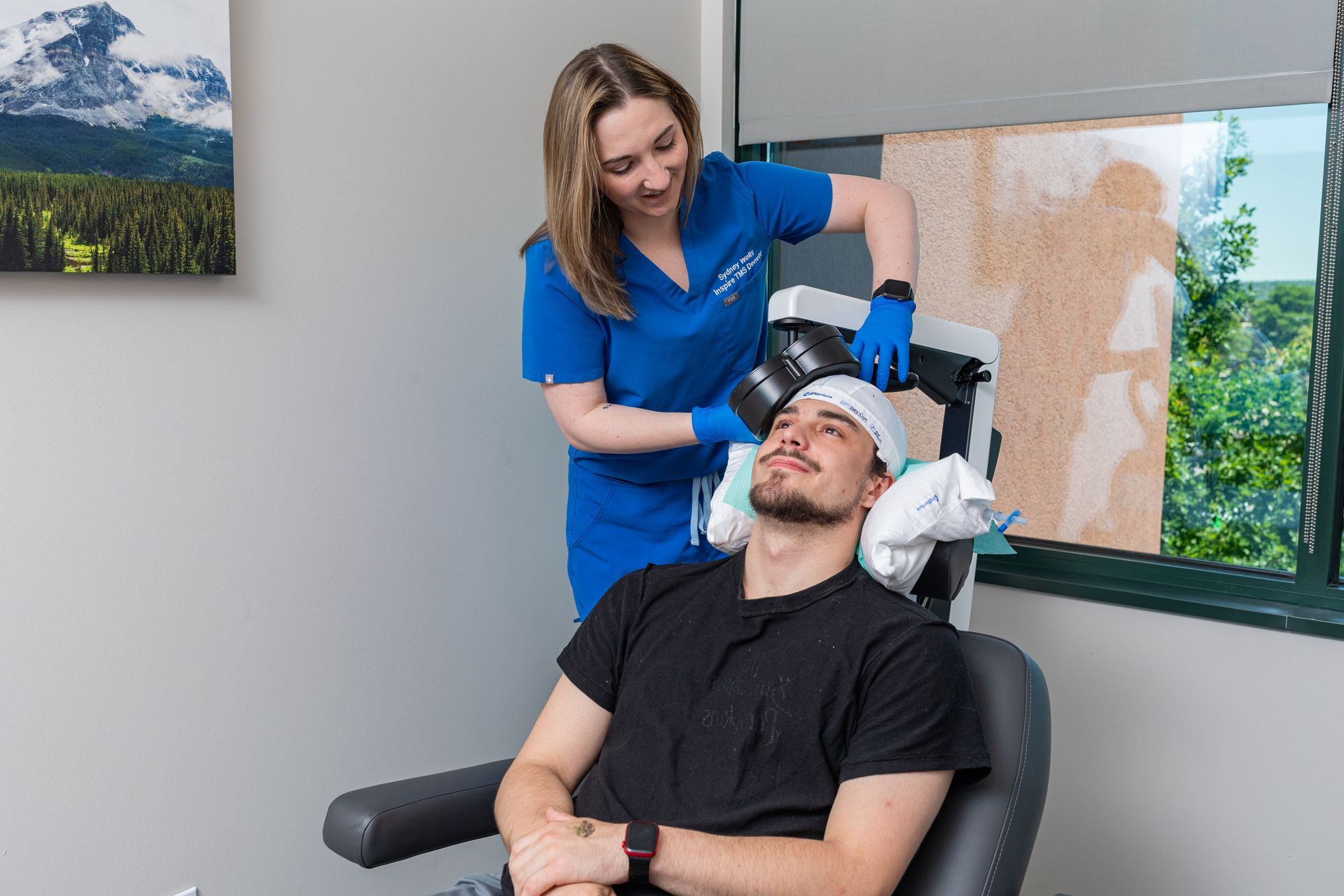
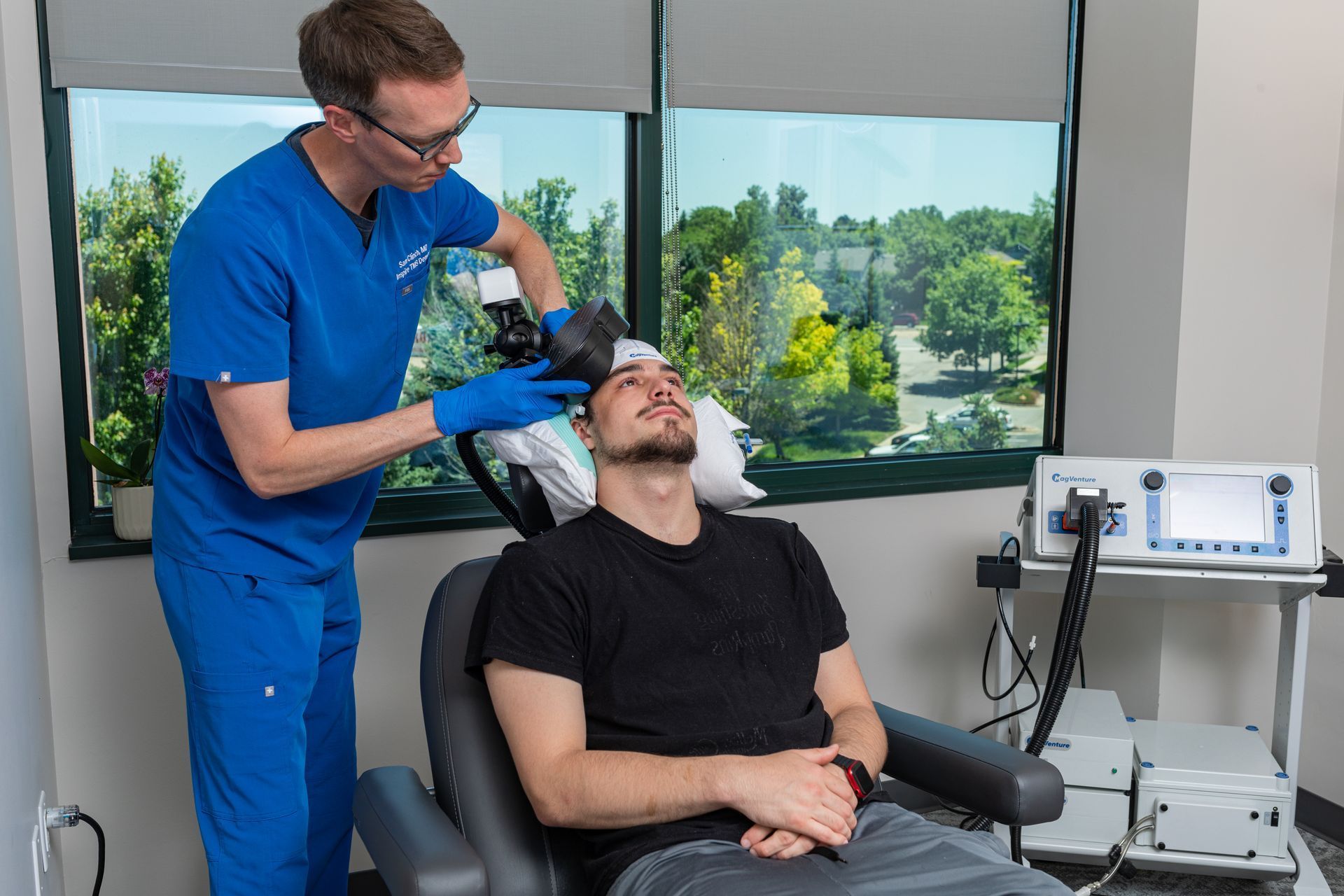
TMS uses a magnetic coil placed over the scalp to directly stimulate specific cortical regions (commonly the dorsolateral prefrontal cortex for depression). Repeated stimulation over days to weeks produces enduring changes in brain circuits (neuroplasticity), helping underactive networks regain healthier patterns. This circuit-based approach is biologically distinct from the way antidepressants modulate neurochemistry, which is one reason TMS can help people whose depression did not respond to medications. TMS is non-invasive, performed outpatients, and does not produce systemic medication side effects.
Evidence: Does TMS help people with TRD?
Yes. Large trials and many real-world clinic outcomes show that TMS can produce meaningful responses and remission in patients with TRD. Clinic-level tracking at Inspire and published meta-analyses report robust response rates among patients who previously failed multiple medication trials. Importantly, many people who respond to an initial TMS course will also benefit from retreatment or maintenance if symptoms recur - so TMS can be both an acute and a long-term tool.

What does a TMS care pathway typically look like?
- Consultation & screening. A TMS-trained psychiatrist reviews your history, past medication trials, suicidality risk, and safety (metal implants, seizure history). Clinics usually collect objective symptom measures (PHQ-9) to track progress.
- Mapping & first session. The physician performs a mapping procedure to find the ideal stimulation site and the right intensity for you. The first treatment often follows mapping.
- Course selection. Standard rTMS courses run daily (weekdays) for ~4–6 weeks; iTBS (theta burst) offers shorter sessions; accelerated protocols compress many sessions into days for some candidates. Your clinician will recommend a plan based on history, logistics and goals.
- Monitoring & maintenance. Progress is tracked and, if needed, maintenance or booster sessions are offered to sustain benefit. Clinics also discuss integrating medication/therapy as part of ongoing care.
Safety & tolerability - what to expect
TMS is generally well tolerated. The most common side effects are transient scalp tenderness or mild headache. Serious events (like seizure) are very rare when screening is done correctly. TMS is routinely favored for patients seeking non-systemic treatment without anesthesia, and clinics explicitly compare its safety and tolerability favorably to more invasive neuromodulation like ECT. Nonetheless, careful screening and ongoing monitoring are standard.
Where TMS fits with other advanced options (ketamine, ECT, residential)
Ketamine / Spravato:
These can provide rapid relief for some patients and are useful in crisis or when rapid reduction of suicidal ideation is needed. However, effects may be transient for some people. TMS can be complementary - ketamine for rapid rescue and TMS for durable circuit retraining. Clinicians often design a sequence or combine approaches depending on needs.
ECT:
Still the most effective option for some severe, refractory depressions, but it requires anesthesia and carries additional recovery considerations. TMS is a less invasive option with an excellent tolerability profile and can be considered for those reluctant to pursue ECT. A clinician will help weigh risks/benefits.
Residential care:
Important for stabilization and intensive psychotherapy. If symptoms persist after residential treatment, TMS is a logical brain-directed next step to consolidate gains or treat residual biological symptoms.
Who is most likely to benefit?
People with clearly documented TRD - those who have tried adequate antidepressant courses (and often psychotherapy) without sufficient response - are primary candidates. Partial responders to other treatments, people unable to tolerate medication side effects, or those who prefer a non-drug intervention are also common candidates.
Adolescents (age 15+) may be considered in selected circumstances with careful evaluation. A psychiatrist experienced in TMS will determine candidacy after a comprehensive review.

Download Your Roadmap to TMS
Want a clear picture of what to expect? Download the TMS Treatment Roadmap by Sydney
What about insurance & access?
Most major commercial insurers,
Medicare and Tricare cover TMS for treatment-resistant major depressive disorder when plan criteria are met and prior authorization is obtained. Coverage details vary - insurers typically require documentation of medication trials, severity measures (PHQ-9), and a psychiatrist’s letter of medical necessity. Accelerated protocols and off-label indications (some anxiety/PTSD uses) are often not covered; clinics offer sliding-scale or self-pay options for those cases. Always ask the clinic to run a benefits check and provide an itemized estimate.
Patient stories & real-world hope
Clinic reviews and patient accounts often describe people who finally achieved meaningful recovery with TMS after long medication trials, ketamine courses, or residential stays. These stories don’t guarantee outcomes, but they reflect clinical experience: TMS can produce substantial, durable improvements for many people who have struggled for years.
Inspire’s outcome tracking supports these real-world successes.
How to explore TMS as a bridge (practical next steps)
- Collect your treatment history. List antidepressants tried (names/dates/doses), any ketamine/Spravato treatments, psychotherapies and residential programs, and recent symptom scores (PHQ-9).
- Request a TMS consult. A TMS psychiatrist will review your record, screen for safety, and explain likely benefits and realistic expectations. Inspire offers phone consultations and runs benefits checks.
- Discuss sequencing. If you’ve had recent ketamine or other interventions, ask how TMS could be sequenced or combined to maximize durability of benefit.
- Plan for mapping & course. If you proceed, expect a mapping session and a personalized plan (standard, iTBS or accelerated) with clear monitoring and a maintenance strategy if you respond.
Learn More: TMS Therapy
I tried many meds and ketamine without lasting benefit. Should I try TMS?
Yes - different biological mechanisms mean TMS can work when other treatments did not. A TMS psychiatrist can assess your history and recommend a personalized plan.
How soon will I feel better?
Responses vary. Some patients notice improvements in 1–3 weeks; many reach substantial benefit by 6–8 weeks. Accelerated protocols may speed effects for selected patients.
Is TMS safe if I’ve had ECT or ketamine?
TMS can be used after other treatments, including ECT and ketamine, but decisions depend on your clinical history. Your psychiatrist will assess safety and sequencing.

Every Question Answered
Want to know more about TMS? Check out this in-depth guide to TMS therapy with transparent and easy to understand explanations about TMS processes, protocols, and treated conditions.
Latest Posts