Can TMS Be Administered at Home?

Exploring the Fisher Wallace Stimulator vs Clinical TMS
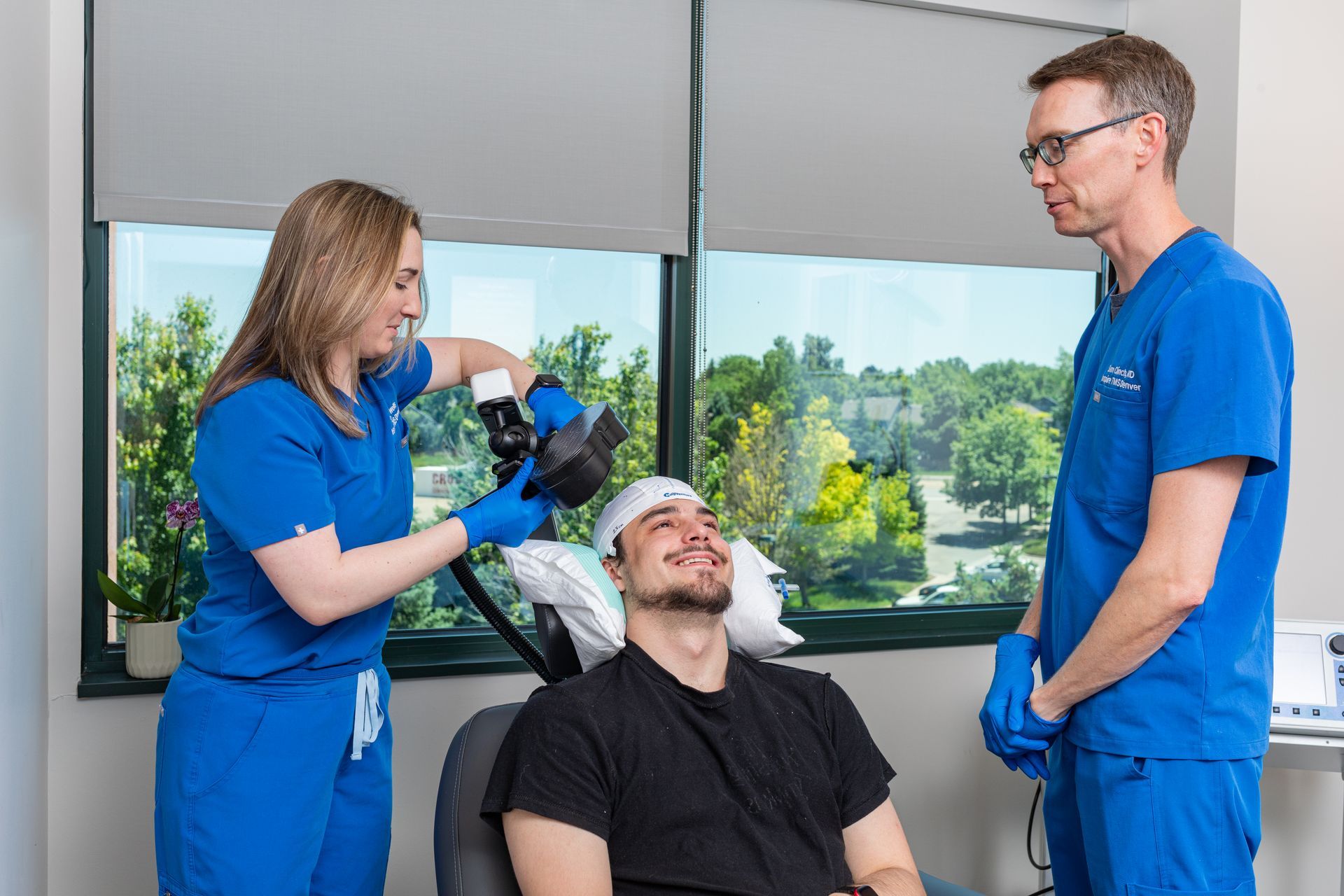
Transcranial Magnetic Stimulation (TMS) is becoming a widely recognized treatment for depression, especially when traditional approaches like medication don’t work. But with the rising cost of living and inconsistent insurance coverage, many people are looking for home-based alternatives.
One such device gaining attention is the Fisher Wallace Stimulator - a product approved by the FDA and marketed as a patient-owned treatment for depression, anxiety, and insomnia.

Not Sure If Insurance Covers TMS?
Get a personalized estimate - see if you qualify for insurance and what you’d pay without coverage.
What Is the Fisher Wallace Stimulator?
The Fisher Wallace Stimulator delivers low-dose electrical waves to general surface areas of the brain. It uses Transcranial Direct Current Stimulation (tDCS), which is a type of neuromodulation - the process of altering nerve activity by delivering electrical stimulation to targeted areas.
In simpler terms, the device mimics the brain stimulation aspect of TMS but in a one-size-fits-all, home-based format. It is non-invasive, used for 20-minute self-guided sessions daily for about a month, and may help:
- Increase serotonin production
- Improve mood and brain connectivity
- Enhance the effects of antidepressants or reduce the need for medication
It’s also being used as a complementary therapy for sleep disorders and anxiety.
TMS vs Fisher Wallace: How Do They Compare?
While both approaches use neuromodulation, the difference in clinical precision and results is significant.
| Fisher Wallace (Home Use) | TMS (In-Clinic Treatment) |
|---|---|
| Electrical stimulation | Magnetic wave stimulation |
| Generalized stimulation across brain surface | Precisely targets brain areas based on individual brain mapping |
| Self-administered, fixed protocol | Supervised, customizable protocols tailored to your condition |
| Early-stage or mild depression support | Proven efficacy in severe, treatment-resistant depression |
| No insurance coverage | Often covered by insurance with medical referral |
| One-size-fits-all | Personalized treatment plans & tracked outcomes |
Is Home Stimulation a Viable Option?
For individuals dealing with mild depression, anxiety, or sleep issues, devices like Fisher Wallace may provide a low-risk, accessible option - especially if you're looking to reduce medication reliance or need a non-invasive solution during pregnancy.
But for moderate to severe depression or treatment-resistant cases, clinical TMS remains the gold standard. It offers:
- Measurable improvement in most patients
- 80%+ response rate at Inspire TMS Denver
- Complete remission in many cases
Final Thoughts
Home neuromodulation tools may have a place in mental health care - particularly for early intervention or maintenance. But they don't match the targeted power, customization, or outcomes of clinical TMS. Want to learn more about your options or determine which path is right for you?
Take our quiz: Is TMS Right for Me? Or book a free 10-minute call with our doctor to talk through your goals and treatment options.

Every Question Answered
Want to know more about TMS? Check out this in-depth guide to TMS therapy with transparent and easy to understand explanations about TMS processes, protocols, and treated conditions.
Latest Posts